

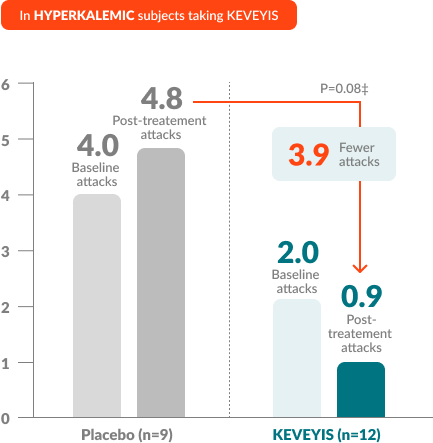
†Treatment effect equals KEVEYIS attack rate per week minus placebo attack rate per week; adjusted for missing diary days. Attacks per week (defined as weekly attack rate) was the average number of self-reported attacks of muscle weakness per week. The posttreatment attacks were calculated as the average number of attacks per week over the final 8 weeks of the 9-week double-blind phase. The first week of diary data was excluded to avoid contamination of the efficacy assessment by the participant’s previous treatment, if any.
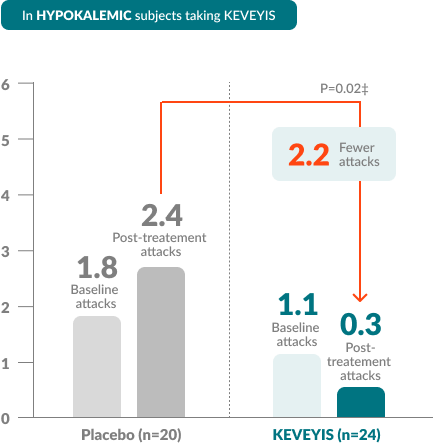
‡Treatment effects in the HyperPP and HypoPP substudies were -4.1 and -2.2, respectively. Treatment effect is computed as the median of the bootstrap distribution of treatment group difference in median response. The 95% confidence interval (CI) is computed using the 2.5 and 97.5 percentiles of this bootstrap distribution.
From the 9-week, double-blind phase of Study 1, 41 of 44 patients in the hypokalemic substudy (KEVEYIS, n=22) and 17 of 21 patients in the hyperkalemic substudy (KEVEYIS, n=9) moved into a 1-year, open-label extension (OLE) phase in which all participants received KEVEYIS.
AR=attack rate; IQR=interquartile range; Wk=week.
AR/Wk, median (IQR)
AR/Wk, median (IQR)
AR/Wk, median (IQR)
AR/Wk, median (IQR)
*During the 1-year, uncontrolled extension phase, outcomes assessed included attack rate, severity-weighted attack rate, total attack duration per week (from weeks 54-61), and changes from week 9 to 61 in measures of strength and lean body mass. Adverse events and other safety outcomes were also considered.
Learn about the experiences of real Primary Periodic Patients who take KEVEYIS.*
28-year-old patient with paramyotonia congenita
Initial treatment: Mexiletine
Patient has been on KEVEYIS and receives no other concomitant medications for PPP. Patient initially reported a 4-week period of intermittent confusion, which resolved spontaneously.
23-year-old patient with hypokalemic PPP
Initial treatment: Acetazolamide
Patient was assessed every 6 months and demonstrated decrease in attack frequency and severity.

(pins and needles)
If you’re ready to prescribe KEVEYIS for a patient, you must start by filling out our Prescription Start Form.
It may take up to 9 weeks to find the right dose for your patient and to assess side effects.
Patients share their experience of taking KEVEYIS for PPP.

Managing Primary Periodic Paralysis (PPP) takes more than medication alone. That’s why we created Xeris CareConnection™, a range of free support services for the PPP community.

KEVEYIS is indicated for the treatment of primary hyperkalemic periodic paralysis, primary hypokalemic periodic paralysis, and related variants.
Concomitant Use of Aspirin or Other Salicylates
Use during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known in humans whether dichlorphenamide is excreted in human milk; exercise caution when administered to a nursing woman.
The most common adverse reactions seen in clinical trials (incidence ≥ 10% and greater than placebo) include paresthesias, cognitive disorder, dysgeusia, and confusional state.
Please see Full Prescribing Information.
Keveyis®, Xeris Pharmaceuticals®, Xeris CareConnection™, and their associated logos are trademarks owned by or licensed to Xeris Pharmaceuticals, Inc.

By submitting this form, I understand I am giving Xeris Pharmaceuticals, Inc., its affiliates, and business partners permission to use the personal information provided in this registration form to contact me by the following methods, but not limited to: mail, email, telephone call or in-person about disease and product information, disease or product-related events, support services, market research, and to share promotional and marketing information. By submitting this form, I consent to these uses and am confirming that I have read and agree to the Xeris Pharmaceuticals® Terms of Use and Privacy Statement. I understand I can unsubscribe by clicking on the unsubscribe link in future communications or by sending a letter with my full contact information (eg, name, address, email, phone, etc) to Xeris CareConnection™ Patient Support Services, 1375 W Fulton Street, Suite 1300, Chicago, IL 60607.