KEVEYIS has been shown to reduce the number, severity, and duration of Primary Periodic Paralysis (PPP) attacks1,2
- Efficacy:
- The efficacy of KEVEYIS has been proven in 2 clinical studies1
- Tolerability:
- KEVEYIS has a demonstrated safety profile1
- Flexible oral dosing:
- KEVEYIS offers patients a range of dosing for an individualized treatment approach1
- Diagnostic & coding information:
- ICD-10 Codes: G72.3 (Periodic Paralysis) or G71.19 (Other specified myotonic disorders)
- ICD-9 Codes: 359.3 (Periodic Paralysis) or 359.29 (Other specified myotonic disorders)
- KEVEYIS NDC: 71090-001-01
Case studies: actual patients with PPP who started taking KEVEYIS*
- Common symptoms leading up to attack include calf pain or muscle cramps
- Weakness in leg and hip muscles—resulting in difficulty walking, tripping, and falling
- Weakness in upper extremities during more severe attacks
- Muscles related to breathing, swallowing, or speaking are never involved
- Typical duration of attacks ranges from 2 to 72 hours
- Typical severity varies from mild (limping when walking) to severe (unable to walk or get up from the floor)
- Attacks began occurring at 3 years of age with a frequency of 2 to 3 a month
- Attacks were often triggered by high-carbohydrate meals or intense physical activity
- Weakness was worse in the mornings and improved throughout the day
- Potassium tablets and acetazolamide prescribed
- Acetazolamide became ineffective in the past 2 years despite escalating dose
- Patient was assessed every 6 months and has demonstrated a decrease in attack frequency and severity
- Cramps and myotonia in the hands
- Fatigue in the lower extremities
- Typical duration of attacks is 5 to 30 minutes
- Typical severity of attacks is moderate
- Patient experienced attacks daily in cold weather months and 3 to 5 times per month in the summer
- Common symptoms leading up to attacks included cramping of the hands and reduced exercise tolerance
- Lingering symptoms included stiffness of the hands
- Mexiletine (150 mg TID) prescribed
- After 4 months, discontinued due to lack of response, flu-like symptoms, and gastroesophageal reflux disease (GERD)
- Patient has been on KEVEYIS and receiving no other concomitant medications for PPP
- Patient initially reported a 4-week period of intermittent confusion which resolved spontaneously
- * Case studies are real patients and were shared by practicing physicians specializing in PPP. Due to HIPAA and privacy laws, first names and initials have been changed to protect patients’ identities.
KEVEYIS helped control the number, severity, and duration of PPP attacks in 2 clinical trials1,2
Study design1:
Study 1 was a randomized, double-blind, placebo-controlled, parallel-group, multi-center, 9-week study that consisted of 2 substudies—hyperkalemic periodic paralysis (n=21) and hypokalemic periodic paralysis (n=44). The primary endpoint was the average number of self-reported attacks of muscle weakness per week during the last 8 weeks of the study. Patients already on KEVEYIS prior to the study continued on the same dose. In patients taking acetazolamide prior to the study, the dose of KEVEYIS was 20% of the acetazolamide dose. Treatment-naive patients received 50 mg of KEVEYIS BID. Dose reduction for tolerability was permitted.Dr. Griggs, a PPP expert, discusses 2 clinical trials in PPP
Please see Important Safety Information and full Prescribing Information available on this website.From baseline to week 9, treatment with KEVEYIS decreased weekly attack rates while treatment with placebo resulted in increases1,2†
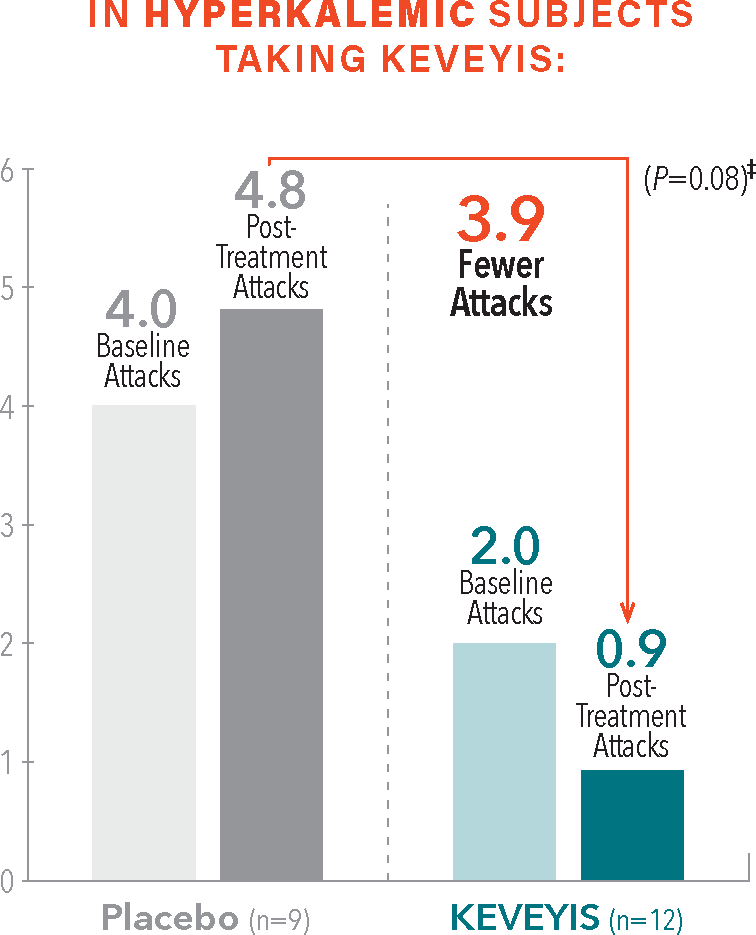
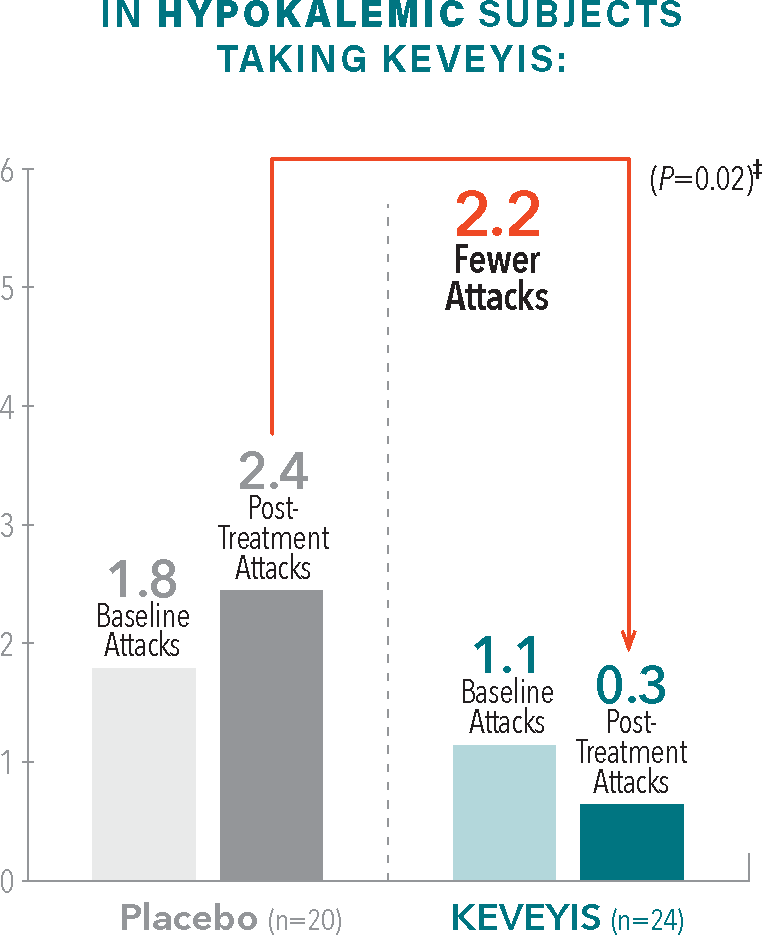
- †Treatment effect equals KEVEYIS attack rate per week minus placebo attack rate per week; adjusted for missing diary days. Attacks per week (defined as weekly attack rate) was the average number of self-reported attacks of muscle weakness per week. The posttreatment attacks were calculated as the average number of attacks per week over the final 8 weeks of the 9-week double-blind phase. The first week of diary data was excluded to avoid contamination of the efficacy assessment by the participant’s previous treatment, if any.2
- ‡Treatment effect is computed as the median of the bootstrap distribution of treatment group difference in median response. The 95% confidence interval (CI) is computed using the 2.5 and 97.5 percentiles of this bootstrap distribution.2
Treatment with KEVEYIS demonstrated a reduction in attack severity vs placebo§
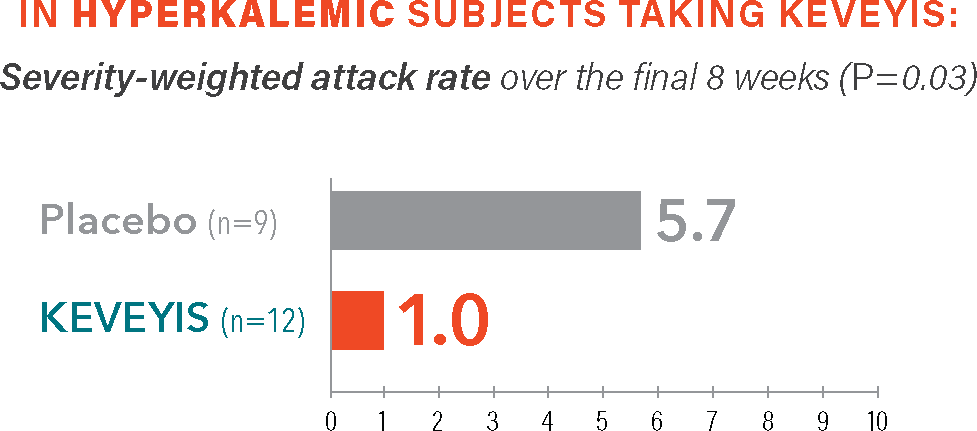
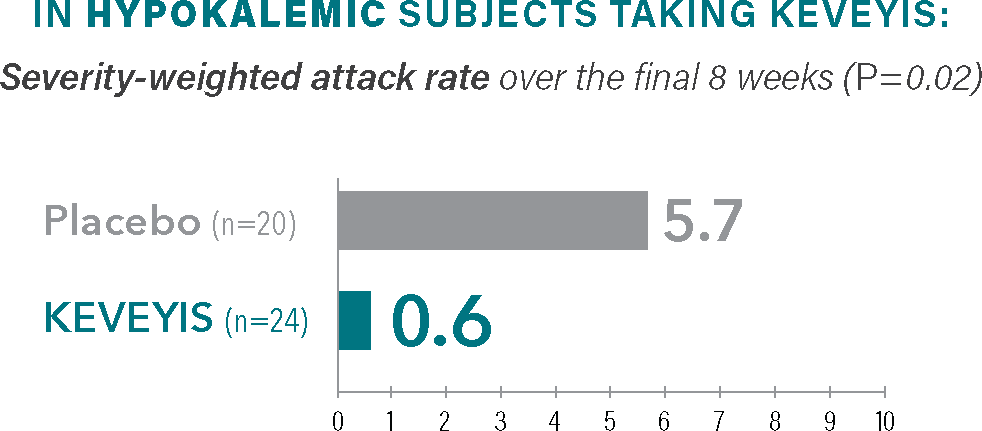
Self-reported measure of attack severity, with 10 being the most severe.§
- §The severity-weighted attack rate was calculated from self-reported scores of attack severity (scored as 1-10 with increasing severity) defined as the sum of average attack severity across all distinct attacks over the final 8 weeks (weeks 2-9) divided by the number of weeks that the patient was followed. Severity-weighted attack data were adjusted for missing diary days.2
Treatment with KEVEYIS shortened weekly attack duration vs placebo||
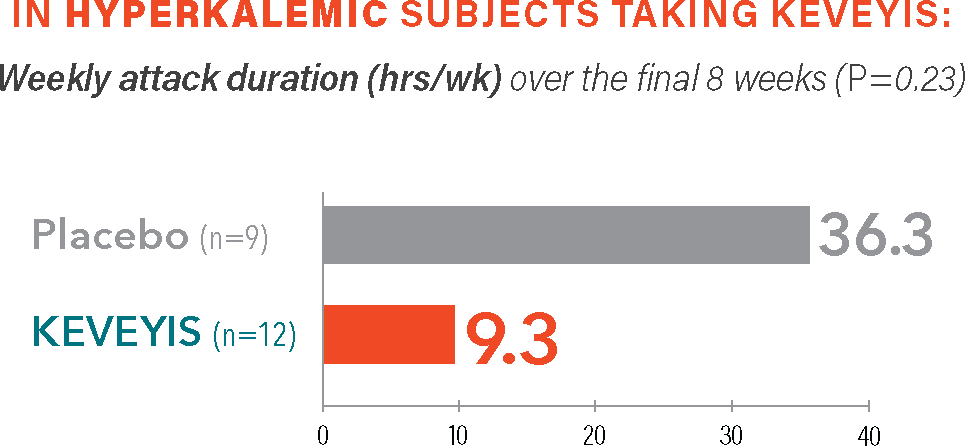
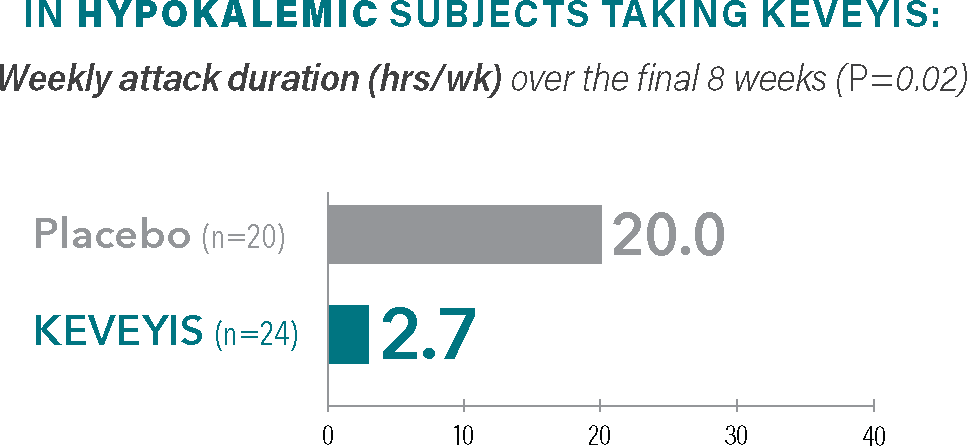
Self-reported attack duration across all distinct attacks (weeks 2-9), represented in total hours.||
- ||The weekly attack duration was calculated from self-reported attack duration across all distinct attacks over the final 8 weeks (weeks 2-9) divided by the number of weeks that the patient was followed. Weekly attack duration was adjusted for missing diary days.2